A "Lung Enema" concept!
VIDEOS of 'Balancing Chariots in Action!
1sr CHARIOT
COSMIC GRASSHOPPER
A Frozen TINMAN
A recent wedding gig!
NY TIMES PHOTOS!
FUTURE Models!
A SK8nHOPPER Concept!
CONTACT INFO
BURNING MAN photos
PERPETUAL MOTION MAN
Many PATENTS!
FOLDABLE CHARIOT!
Our OCEAN OF AIR!
The Gooey Message Down Under!
ALL WRAPPED UP IN BLUE!
Welcome to GULLIVER
Birds,Frogs,Fish & Fresh Air
DREAMCATCHER SWINGS
The CRYOPERK Engine
Rickshaw/GRANNY TRIKE!
Charioteering PHYSICS!
AIRGULPER
HEALING CELLPHONES!
A FLOATIN' FORD!
SWINGSET Power
A "LUNG ENEMA"!
Crystal Yurt GREENHOUSE
SPINNING AIRSHIP GLIDER
SANTA BARBARA Chariot
WHALE'S TAIL Wind Energy Harvester
A 'SNOWHOPPER' Concept!
Let's Go Phi a Kite!
Christmas Eve
VENICEbeachHOPPIN'
FLOAT To Space...
Electric Chariot Dreamin'
NASA Satellite Re-Entry Danger
Monster Mars Rocket
PINECONEspinningAirship / FanwingSOLARSHIP
Oxygenating, Detoxifying, Mosquito-Repelling AEROPONICS
A Festive PUPPETHOPPER
OH THE SITES...
A ONE-WHEELED Chariot!
Some FACTUAL ERRORS of the SPACE PROGRAM
In Depth FANWING
Charioteering Lifestyle
The "'SpacedOutX' Dragon V2"
(Scroll down to the bottom for pictures)
Liquid breathing is a form of respiration in which a normally air-breathing organism breathes an oxygen rich liquid (usually from the perfluorocarbon family), rather than breathing air. It is used for medical treatment and could someday find use in deep diving and space travel. Liquid breathing is sometimes called "fluid breathing", but this is misleading as both liquids and gases are fluids.
The early experiments
In the mid-1960s Dr. J. Kylstra, a physiologist at the University at Buffalo, realized that salt solutions could be saturated with oxygen at high pressures. In a US Navy recompression chamber, Kylstra experimented to see if mice could move the saline solution in and out of their lungs, while extracting enough oxygen from the fluid to survive. The mice and rats could breathe the liquid (he could keep the animals alive for up to 18 hours), but carbon dioxide was not removed fast enough from the system, and quickly built up to near-toxic levels. This had to be fixed before liquid breathing could be used in humans.
In 1966 Dr. Leland Clark and Dr. Golan experimented on liquid breathing in mice. Oxygen and carbon dioxide are very soluble in fluorocarbon liquids such as freon. Leland Clark realized that, if the alveoli of the lungs can draw oxygen out of the liquid and unload carbon dioxide into the liquid, these fluorocarbons should support respiration of animals. Testing first on anesthetized mice, he temporarily paralyzed each animal and put a tube down its trachea, inflating a cuff inside the airway to provide a seal and ensure that no air entered the lungs, and no solution leaked out[1].
After bubbling oxygen through the fluorocarbon, the oxygenated fluid was pumped into the animals' lungs, and recirculated at about 6 cycles of inhalation and exhalation per minute. Most of the animals who were kept in the fluid for up to an hour survived for several weeks after their removal, before eventually succumbing to pulmonary damage. Autopsies uniformly revealed that the lungs appeared congested when collapsed but normal when inflated.
As in Kylstra's studies, Clark had problems due to the size of the animals' airways. The tiny size limited the amount of fluid that could get into the lungs. For that and other reasons, carbon dioxide tended to build up in the system and could not be removed fast enough. Dr. Clark discovered that the length of time the mice could survive in the fluid was directly related to the fluorocarbon's temperature: the colder the fluid, the lower the respiration rate, which prevented carbon dioxide buildup. The only way was to induce hypothermia in the animals. This technique seemed to give him the most success, as one animal survived over 20 hours breathing fluid at 18 ºC.
All animals in the earliest studies suffered lung damage, but whether that was due to toxic impurities in the fluorocarbon, chemical interaction of the fluorocarbon with the lung, or some unknown effect, was undetermined. This mystery of the lung damage, and the problem of carbon dioxide elimination, and the body tissues tending to retain the fluorocarbon, would have to be solved before the process could be attempted on human subjects. Also, perfluorocarbon is denser and more viscous than air. This increases resistance and thus the effort needed to breathe.
Later developments
During later years, the techniques of fluid breathing were constantly refined and improved. The survival rate of all the tested animals in recent years has been very high, thanks mainly to improvements in carbon dioxide elimination. Current fluids used can dissolve over 65 ml of oxygen and 228 ml of carbon dioxide per 100 ml perfluorocarbon. By the early 1990s this procedure developed:
The animal was anesthetized with intravenous sodium thiopental.
The animal was put on its back. A tube was placed down its airway, ready for the liquid breathing medium.
A blood sample was taken. The temperature of the fluid was adjusted correspondingly. It was no longer necessary to make the animals hypothermic.
The perfluorocarbon was instilled into the animal's lungs through the tube.
A floor-mounted 3-litre reservoir was filled with the perfluorocarbon. The liquid was driven by a pump through a series of machines which warmed and oxygenated the liquid and took the carbon dioxide out of it. The liquid flowed through a tube into a 3-way pneumatic valve which directed flow to the animal. A computer controlled the inspiration (18 ml of fluid per second), pumping the liquid into the animal's lungs, then back out again to the reservoir, at a rate of about 6 complete respirations per minute.
At the end of the test, the animal was tilted for about 15 seconds and the perfluorocarbon was allowed to drain from the lungs. This can be seen in the film The Abyss where Ensign Monk drained the fluid out of the rat's lungs: in the filming, the rat genuinely breathed liquid.
These tests of the early 90s were successful: dogs could be kept alive in the perfluorcarbon medium for about 2 hours; after removal the dogs were usually slightly hypoxic, but returned to normal after a few days. When the animals were autopsied, the typical findings were mild oedema and some hemorrhaging, clearly an improvement over the lung damage of earlier tests.
Use in diving
To meet Wikipedia's quality standards, this article or section may require cleanup.
Please discuss this issue on the talk page, or replace this tag with a more specific message. Editing help is available.
Breathing liquid instead of air seems odd, but if the technique could be perfected it would revolutionize diving.
In diving, the pressure inside the lungs must equal the pressure outside the body, otherwise the lungs collapse. Thus, if the diver is f feet or m meters deep, and the air pressure at the water surface is p bars (usually p = 1, but it is less at high-altitude lakes such as Lake Titicaca), he must breathe fluid at a pressure of f/33+p = m/10+p bars. This pressure quickly gets high with depth: around 13 bars at 400 feet (120m), and around 500 bars on the oceans' abyssal plains. These high pressures cause harmful effects on the body, especially when quickly released: air emboli and other diving disorders, like nitrogen narcosis and decompression sickness. One solution is a rigid articulated diving suit, but these are bulky and clumsy. A more moderate option to deal with narcosis is to breathe heliox or trimix, in which some or all of the nitrogen is replaced by helium. However, this option does not deal with the problem of bubbles and decompression sickness, because helium dissolves in tissues and causes bubbles when pressures are released, just like nitrogen does.
With liquid in the lungs, the pressure in our body could accommodate changes in the pressure of the surrounding water without the huge gas partial pressure exposures required when the lungs are filled with gas. The elimination of gasses at high partial pressures would eliminate saturation of the body's tissues with high pressure nitrogen or helium, and thus the need for slow decompression and its above inherent problems to prevent decompression sickness. (Diving mammals, as well has free-diving humans who dive to great depths on a single breath, have little or no problem with decompression sickness with rapid decompression on surface-return, since a single breath of gas does not contain enough total nitrogen to cause tissue bubbles on decompression. In very deep-diving mammals and deep free-diving humans, the lungs almost completely collapse).
If the technique of total liquid ventilation for divers could be perfected, it would be extremely useful for submarine escape and undersea oxygen support facilities, and for underwater work, as portrayed in the 1989 science-fiction film The Abyss.
Unfortunately, there are problems with execution of the idea. All uses of liquid breathing for diving must involve total liquid ventilation. Any bubbles in the system at all would need to be maintained by the kinds of high partial gas pressures that it is the whole point of the system to avoid. Bubbles would lead to bends. Total liquid ventilation, however, has difficulty moving enough fluid to carry away CO2, because no matter how great the total pressure is, the amount of partial CO2 gas pressure available to disolve CO2 into the breathing liquid can never be much more than the pressure of 40 mm of mercury (Torr) at which CO2 exists in the blood. At these pressures, most fluorocarbon liquids require about 70 mL/kg minute-venilation volumes of liquid (about 5 L/min for a 70 kg adult) to remove enough CO2 for normal resting metabolism. This is a great deal of fluid to move when it represents dense liquid (about 1.8 times the density of water), and even moderate work which doubles CO2 production, would double this figure. It seems unlikely that a person would move 10 liters/min of fluorocarbon liquid without assistance from a mechanical ventilator, so "free breathing" of liquids by working human aquanauts as seen in the film The Abyss, will probably be a long time coming.
Medical uses
The immediate use of liquid breathing is likely to be in treating premature babies, and adults with severe lung damage from causes such as fires.
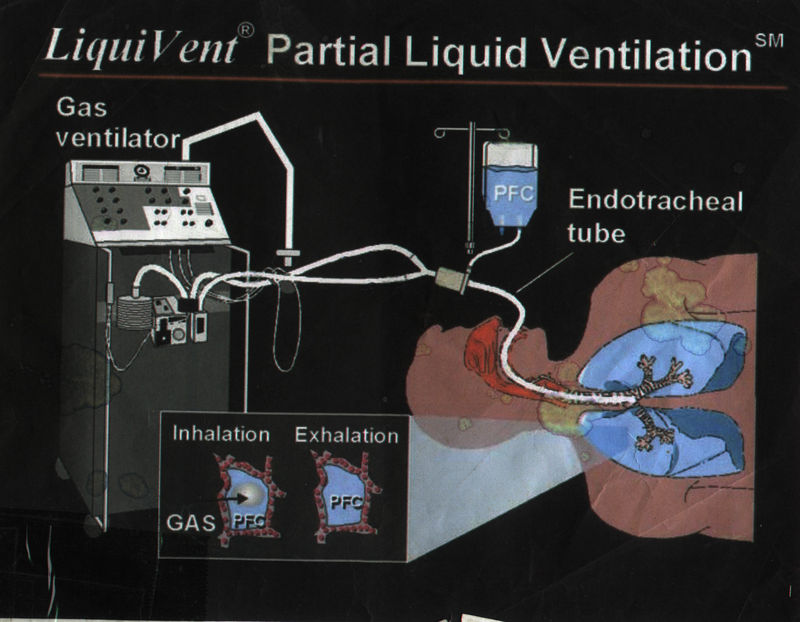
Liquid breathing began to be used by the medical community after the development by Alliance Pharmaceuticals of the fluorochemical perfluorooctyl bromide, or perflubron for short. Useful as a blood substitute and for liquid ventilation, perflubron (under Alliance Pharmaceutical's brand name LiquiVent) is instilled directly into the lungs of patients with acute respiratory failure (caused by infection, severe burns, inhalation of toxic substances, and premature birth), whose air sacs have collapsed. Once inside the lungs, perflubron enables collapsed alveoli (air sacs) to open and permits a more efficient transport of oxygen and carbon dioxide. Current tests are focusing on premature babies, but trials with adults are ongoing.
All blood that flows out from the heart to the rest of the body first must go through the lungs, where it picks up oxygen and gets rid of carbon dioxide. If the lungs do not function properly, as is common in premature infants with respiratory distress syndrome, the lungs become stiff and collapse, and the infants must be put on ventilators. A study, led by Dr. Corrinne Leach of the University at Buffalo, tested 13 infants on ventilators who were born prematurely with respiratory distress syndrome. The infants were at risk of dying because they could not produce a natural surfactant that stops the lungs from collapsing from surface tension. They were at risk of severe and permanent lung damage from the force of the ventilators that were inflating their lungs. Their lungs were filled with perflubron which would let the air sacs of the lungs open and permit breathing. The perflubron let the lungs inflate with less pressure and let oxygen pass through the lungs and into the blood stream and carbon dioxide out more efficiently and with less stress. This was successful.
The 13 premature infants received partial liquid ventilation for 24 to 76 hours; they were weaned back to gas ventilation without difficulties or adverse side effects, and 11 of the 13 showed significant improvement in lung functioning. Six of the infants eventually died, but of causes apparently unrelated to the liquid ventilation.[2]
Clinical trials with premature infants, children and adults were conducted. Since the safety of the procedure and the effectiveness of the gas exchange have improved so much, the US Food and Drug Administration (FDA) gave the product "fast track" status (meaning an accelerated review of the product, designed to get it to the public as quickly as is safely possible) due to its life-saving potential. Unfortunately, results of the clinical trials were disappointing and Alliance is no longer pursuing partial liquid ventilation application.
Mode of application
Despite recent advances in liquid ventilation, a standard mode of application of perfluorocarbon (PFC) has not been established yet.
TLV
Although total liquid ventilation (TLV) with completely liquid filled lungs is beneficial, the necessity for a liquid filled tube system that contains pumps, heater and membrane oxygenator to deliver and remove tidal volume aliquots of conditioned perfluorocarbon to the lungs is of great disadvantage.
PLV
In contrast, partial liquid ventilation (PLV) can be applied using standard ventilators connected with gas filled standard respirator systems, delivering tidal volumes of oxygen-air mixture to perfluorocarbon filled lungs. The influence of PLV on oxygenation, carbon dioxide removal and lung mechanics has been investigated in several animal studies using different models of lung injury. Clinical applications of PLV have been reported in patients with acute respiratory distress syndrome (ARDS), meconium aspiration syndrome, congenital diaphragmatic hernia and respiratory distress syndrome (RDS) of neonates. PLV requires extreme respiratory care, because the ventilatory setting is determined by the perfluorocarbon filled lung. Profound expertise is mandatory to perform and maintain filling of the lung with perfluorocarbon to functional residual capacity (FRC). Disruption of PLV immediately deteriorates gas exchange. Incomplete filling of the lung has been shown to be less effective than filling the lung to functional residual capacity volume. Severe adverse events affecting gas exchange and pulmonary circulation limit the use of PLV.
New application modes for PFC have been developed[3].
PFC vapor
Vaporization of perfluorohexane with two anesthetic vaporizers calibrated for perfluorohexane has been shown to improve gas exchange in oleic acid induced lung injury in sheep [4]. Predominantly PFCs with high vapor pressure are suitable for vaporization.
Aerosol-PFC
With aerosolized perfluorooctane, significant improvement of oxygenation and pulmonary mechanics was shown in adult sheep with oleic acid-induced lung injury. In surfactant-depleted piglets, persistent improvement of gas exchange and lung mechanics was demonstrated with Aerosol-PFC [5]. The aerosol device is of decisive importance for the efficacy of PFC aerosolization, as aerosolization of PF5080 (a less purified FC77) has been shown to be ineffective using a different aerosol device in surfactant-depleted rabbits (Kelly). Partial liquid ventilation and Aerosol-PFC reduced pulmonary inflammatory response [6].
Space travel
Around 1970, liquid breathing found its way into fiction, in alien spacesuits in the Gerry Anderson UFO series, which enabled a spaceman to withstand extreme acceleration forces.
Forces applied to fluids (such as gravitational forces on Earth) are distributed as omnidirectional pressures. This fact is fundamental to all hydraulics. In the ocean, this distribution of force allows organisms such as whales to grow to sizes that would be unsupportable on dry land.
Because liquids are incompressible, they do not change density under high acceleration such as performed in aerial maneuvers or space travel. A man immersed in such a liquid would have inertial forces distributed around his body, rather than applied at a single point such as a seat or harness straps. The effect of high acceleration is caused by different parts of the accelerating volume having different densities and thus different momentums, and with acceleration in air in the spaceman's body has a very different density from the air or vacuum in the spacecraft; as a result, the effect is much less if he is immersed in liquid and is breathing liquid. (Liquid breathing in deep diving is for a different purpose: to avoid the physiological effects of breathing high-pressure gas: see Diving hazards and precautions.)
However, such application is probably physically and anatomically not possible. The main problem with acceleration forces is that they force the heart to pump blood at much higher pressures. Liquid breathing would not change that. Moreover, filling lungs with liquid, especially as heavy as perflurocarbon, will dramatically increase their weight. At extreme G forces, experienced by pilots and astronauts, the lungs are likely to rupture if filled with liquid.
The early experiments
In the mid-1960s Dr. J. Kylstra, a physiologist at the University at Buffalo, realized that salt solutions could be saturated with oxygen at high pressures. In a US Navy recompression chamber, Kylstra experimented to see if mice could move the saline solution in and out of their lungs, while extracting enough oxygen from the fluid to survive. The mice and rats could breathe the liquid (he could keep the animals alive for up to 18 hours), but carbon dioxide was not removed fast enough from the system, and quickly built up to near-toxic levels. This had to be fixed before liquid breathing could be used in humans.
In 1966 Dr. Leland Clark and Dr. Golan experimented on liquid breathing in mice. Oxygen and carbon dioxide are very soluble in fluorocarbon liquids such as freon. Leland Clark realized that, if the alveoli of the lungs can draw oxygen out of the liquid and unload carbon dioxide into the liquid, these fluorocarbons should support respiration of animals. Testing first on anesthetized mice, he temporarily paralyzed each animal and put a tube down its trachea, inflating a cuff inside the airway to provide a seal and ensure that no air entered the lungs, and no solution leaked out[1].
After bubbling oxygen through the fluorocarbon, the oxygenated fluid was pumped into the animals' lungs, and recirculated at about 6 cycles of inhalation and exhalation per minute. Most of the animals who were kept in the fluid for up to an hour survived for several weeks after their removal, before eventually succumbing to pulmonary damage. Autopsies uniformly revealed that the lungs appeared congested when collapsed but normal when inflated.
As in Kylstra's studies, Clark had problems due to the size of the animals' airways. The tiny size limited the amount of fluid that could get into the lungs. For that and other reasons, carbon dioxide tended to build up in the system and could not be removed fast enough. Dr. Clark discovered that the length of time the mice could survive in the fluid was directly related to the fluorocarbon's temperature: the colder the fluid, the lower the respiration rate, which prevented carbon dioxide buildup. The only way was to induce hypothermia in the animals. This technique seemed to give him the most success, as one animal survived over 20 hours breathing fluid at 18 ºC.
All animals in the earliest studies suffered lung damage, but whether that was due to toxic impurities in the fluorocarbon, chemical interaction of the fluorocarbon with the lung, or some unknown effect, was undetermined. This mystery of the lung damage, and the problem of carbon dioxide elimination, and the body tissues tending to retain the fluorocarbon, would have to be solved before the process could be attempted on human subjects. Also, perfluorocarbon is denser and more viscous than air. This increases resistance and thus the effort needed to breathe.
Later developments
During later years, the techniques of fluid breathing were constantly refined and improved. The survival rate of all the tested animals in recent years has been very high, thanks mainly to improvements in carbon dioxide elimination. Current fluids used can dissolve over 65 ml of oxygen and 228 ml of carbon dioxide per 100 ml perfluorocarbon. By the early 1990s this procedure developed:
The animal was anesthetized with intravenous sodium thiopental.
The animal was put on its back. A tube was placed down its airway, ready for the liquid breathing medium.
A blood sample was taken. The temperature of the fluid was adjusted correspondingly. It was no longer necessary to make the animals hypothermic.
The perfluorocarbon was instilled into the animal's lungs through the tube.
A floor-mounted 3-litre reservoir was filled with the perfluorocarbon. The liquid was driven by a pump through a series of machines which warmed and oxygenated the liquid and took the carbon dioxide out of it. The liquid flowed through a tube into a 3-way pneumatic valve which directed flow to the animal. A computer controlled the inspiration (18 ml of fluid per second), pumping the liquid into the animal's lungs, then back out again to the reservoir, at a rate of about 6 complete respirations per minute.
At the end of the test, the animal was tilted for about 15 seconds and the perfluorocarbon was allowed to drain from the lungs. This can be seen in the film The Abyss where Ensign Monk drained the fluid out of the rat's lungs: in the filming, the rat genuinely breathed liquid.
These tests of the early 90s were successful: dogs could be kept alive in the perfluorcarbon medium for about 2 hours; after removal the dogs were usually slightly hypoxic, but returned to normal after a few days. When the animals were autopsied, the typical findings were mild oedema and some hemorrhaging, clearly an improvement over the lung damage of earlier tests.
Use in diving
To meet Wikipedia's quality standards, this article or section may require cleanup.
Please discuss this issue on the talk page, or replace this tag with a more specific message. Editing help is available.
Breathing liquid instead of air seems odd, but if the technique could be perfected it would revolutionize diving.
In diving, the pressure inside the lungs must equal the pressure outside the body, otherwise the lungs collapse. Thus, if the diver is f feet or m meters deep, and the air pressure at the water surface is p bars (usually p = 1, but it is less at high-altitude lakes such as Lake Titicaca), he must breathe fluid at a pressure of f/33+p = m/10+p bars. This pressure quickly gets high with depth: around 13 bars at 400 feet (120m), and around 500 bars on the oceans' abyssal plains. These high pressures cause harmful effects on the body, especially when quickly released: air emboli and other diving disorders, like nitrogen narcosis and decompression sickness. One solution is a rigid articulated diving suit, but these are bulky and clumsy. A more moderate option to deal with narcosis is to breathe heliox or trimix, in which some or all of the nitrogen is replaced by helium. However, this option does not deal with the problem of bubbles and decompression sickness, because helium dissolves in tissues and causes bubbles when pressures are released, just like nitrogen does.
With liquid in the lungs, the pressure in our body could accommodate changes in the pressure of the surrounding water without the huge gas partial pressure exposures required when the lungs are filled with gas. The elimination of gasses at high partial pressures would eliminate saturation of the body's tissues with high pressure nitrogen or helium, and thus the need for slow decompression and its above inherent problems to prevent decompression sickness. (Diving mammals, as well has free-diving humans who dive to great depths on a single breath, have little or no problem with decompression sickness with rapid decompression on surface-return, since a single breath of gas does not contain enough total nitrogen to cause tissue bubbles on decompression. In very deep-diving mammals and deep free-diving humans, the lungs almost completely collapse).
If the technique of total liquid ventilation for divers could be perfected, it would be extremely useful for submarine escape and undersea oxygen support facilities, and for underwater work, as portrayed in the 1989 science-fiction film The Abyss.
Unfortunately, there are problems with execution of the idea. All uses of liquid breathing for diving must involve total liquid ventilation. Any bubbles in the system at all would need to be maintained by the kinds of high partial gas pressures that it is the whole point of the system to avoid. Bubbles would lead to bends. Total liquid ventilation, however, has difficulty moving enough fluid to carry away CO2, because no matter how great the total pressure is, the amount of partial CO2 gas pressure available to disolve CO2 into the breathing liquid can never be much more than the pressure of 40 mm of mercury (Torr) at which CO2 exists in the blood. At these pressures, most fluorocarbon liquids require about 70 mL/kg minute-venilation volumes of liquid (about 5 L/min for a 70 kg adult) to remove enough CO2 for normal resting metabolism. This is a great deal of fluid to move when it represents dense liquid (about 1.8 times the density of water), and even moderate work which doubles CO2 production, would double this figure. It seems unlikely that a person would move 10 liters/min of fluorocarbon liquid without assistance from a mechanical ventilator, so "free breathing" of liquids by working human aquanauts as seen in the film The Abyss, will probably be a long time coming.
Medical uses
The immediate use of liquid breathing is likely to be in treating premature babies, and adults with severe lung damage from causes such as fires.
Liquid breathing began to be used by the medical community after the development by Alliance Pharmaceuticals of the fluorochemical perfluorooctyl bromide, or perflubron for short. Useful as a blood substitute and for liquid ventilation, perflubron (under Alliance Pharmaceutical's brand name LiquiVent) is instilled directly into the lungs of patients with acute respiratory failure (caused by infection, severe burns, inhalation of toxic substances, and premature birth), whose air sacs have collapsed. Once inside the lungs, perflubron enables collapsed alveoli (air sacs) to open and permits a more efficient transport of oxygen and carbon dioxide. Current tests are focusing on premature babies, but trials with adults are ongoing.
All blood that flows out from the heart to the rest of the body first must go through the lungs, where it picks up oxygen and gets rid of carbon dioxide. If the lungs do not function properly, as is common in premature infants with respiratory distress syndrome, the lungs become stiff and collapse, and the infants must be put on ventilators. A study, led by Dr. Corrinne Leach of the University at Buffalo, tested 13 infants on ventilators who were born prematurely with respiratory distress syndrome. The infants were at risk of dying because they could not produce a natural surfactant that stops the lungs from collapsing from surface tension. They were at risk of severe and permanent lung damage from the force of the ventilators that were inflating their lungs. Their lungs were filled with perflubron which would let the air sacs of the lungs open and permit breathing. The perflubron let the lungs inflate with less pressure and let oxygen pass through the lungs and into the blood stream and carbon dioxide out more efficiently and with less stress. This was successful.
The 13 premature infants received partial liquid ventilation for 24 to 76 hours; they were weaned back to gas ventilation without difficulties or adverse side effects, and 11 of the 13 showed significant improvement in lung functioning. Six of the infants eventually died, but of causes apparently unrelated to the liquid ventilation.[2]
Clinical trials with premature infants, children and adults were conducted. Since the safety of the procedure and the effectiveness of the gas exchange have improved so much, the US Food and Drug Administration (FDA) gave the product "fast track" status (meaning an accelerated review of the product, designed to get it to the public as quickly as is safely possible) due to its life-saving potential. Unfortunately, results of the clinical trials were disappointing and Alliance is no longer pursuing partial liquid ventilation application.
Mode of application
Despite recent advances in liquid ventilation, a standard mode of application of perfluorocarbon (PFC) has not been established yet.
TLV
Although total liquid ventilation (TLV) with completely liquid filled lungs is beneficial, the necessity for a liquid filled tube system that contains pumps, heater and membrane oxygenator to deliver and remove tidal volume aliquots of conditioned perfluorocarbon to the lungs is of great disadvantage.
PLV
In contrast, partial liquid ventilation (PLV) can be applied using standard ventilators connected with gas filled standard respirator systems, delivering tidal volumes of oxygen-air mixture to perfluorocarbon filled lungs. The influence of PLV on oxygenation, carbon dioxide removal and lung mechanics has been investigated in several animal studies using different models of lung injury. Clinical applications of PLV have been reported in patients with acute respiratory distress syndrome (ARDS), meconium aspiration syndrome, congenital diaphragmatic hernia and respiratory distress syndrome (RDS) of neonates. PLV requires extreme respiratory care, because the ventilatory setting is determined by the perfluorocarbon filled lung. Profound expertise is mandatory to perform and maintain filling of the lung with perfluorocarbon to functional residual capacity (FRC). Disruption of PLV immediately deteriorates gas exchange. Incomplete filling of the lung has been shown to be less effective than filling the lung to functional residual capacity volume. Severe adverse events affecting gas exchange and pulmonary circulation limit the use of PLV.
New application modes for PFC have been developed[3].
PFC vapor
Vaporization of perfluorohexane with two anesthetic vaporizers calibrated for perfluorohexane has been shown to improve gas exchange in oleic acid induced lung injury in sheep [4]. Predominantly PFCs with high vapor pressure are suitable for vaporization.
Aerosol-PFC
With aerosolized perfluorooctane, significant improvement of oxygenation and pulmonary mechanics was shown in adult sheep with oleic acid-induced lung injury. In surfactant-depleted piglets, persistent improvement of gas exchange and lung mechanics was demonstrated with Aerosol-PFC [5]. The aerosol device is of decisive importance for the efficacy of PFC aerosolization, as aerosolization of PF5080 (a less purified FC77) has been shown to be ineffective using a different aerosol device in surfactant-depleted rabbits (Kelly). Partial liquid ventilation and Aerosol-PFC reduced pulmonary inflammatory response [6].
Space travel
Around 1970, liquid breathing found its way into fiction, in alien spacesuits in the Gerry Anderson UFO series, which enabled a spaceman to withstand extreme acceleration forces.
Forces applied to fluids (such as gravitational forces on Earth) are distributed as omnidirectional pressures. This fact is fundamental to all hydraulics. In the ocean, this distribution of force allows organisms such as whales to grow to sizes that would be unsupportable on dry land.
Because liquids are incompressible, they do not change density under high acceleration such as performed in aerial maneuvers or space travel. A man immersed in such a liquid would have inertial forces distributed around his body, rather than applied at a single point such as a seat or harness straps. The effect of high acceleration is caused by different parts of the accelerating volume having different densities and thus different momentums, and with acceleration in air in the spaceman's body has a very different density from the air or vacuum in the spacecraft; as a result, the effect is much less if he is immersed in liquid and is breathing liquid. (Liquid breathing in deep diving is for a different purpose: to avoid the physiological effects of breathing high-pressure gas: see Diving hazards and precautions.)
However, such application is probably physically and anatomically not possible. The main problem with acceleration forces is that they force the heart to pump blood at much higher pressures. Liquid breathing would not change that. Moreover, filling lungs with liquid, especially as heavy as perflurocarbon, will dramatically increase their weight. At extreme G forces, experienced by pilots and astronauts, the lungs are likely to rupture if filled with liquid.
The Abyss
Rated PG-13
Directed by:
James Cameron
Starring:
Ed Harris
Mary Elizabeth Mastrantonio
"What is all this stuff?"
"Fluid-breathing system, we just got them. You use it when you go really deep."
"How deep?"
"Deep."
"HOW deep?"
"It's classified. Anyway, you breathe liquid so you can't get compressed. The pressure doesn't get you."
"You mean you got liquid in your lungs?"
"Oxygenated fluorocarbon emulsion."
Click on liquid-breathing mouse to see the Lung Enema patent.
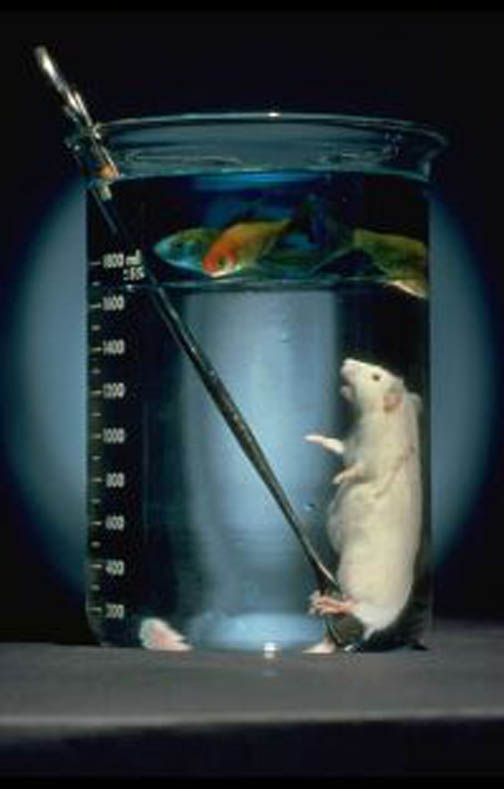
The first real success in fluid breathing came in 1966, with Dr. Leland Clark's "liquid-breathing-mouse" experiment. Dr. Clark (inventor of the Clark electrode) realized that oxygen and carbon dioxide were very soluble in fluorocarbon liquids (like freon). Assuming that the alveoli of the lungs should be capable of drawing oxygen out of the fluid and replacing it with carbon dioxide, Clark suggested that these fluorocarbons should support respiration of animals. Performing the first tests on anaesthetized mice, Dr. Clark temporarily paralyzed each intubated animal, inflating a cuff inside the trachea to provide a seal and ensure that no air entered the lungs, and no solution leaked out.
After bubbling oxygen through the fluorocarbon, the oxygenated fluid was pumped into the animals' lungs, and recirculated (about 6 cycles of inhalation and exhalation per minute). Most of the animals who were kept in the fluid for up to an hour survived for several weeks after their removal, before eventually succumbing to pulmonary damage. Autopsies uniformly revealed that the lungs appeared congested when collapsed but normal when inflated. Some of the early problems Clark encountered seemed to be due to the size of the animals' airway. The tiny size physically limited the amount of fluid that could get into the lungs. For that and other reasons, carbon dioxide tended to build up in the system: it simply couldn't be removed fast enough.
This photograph demonstrates a living mouse breathing in the liquid, while a goldfish inhabits the water floating on top.
Dr. Clark discovered that the length of time the mice could survive in the fluid was directly related to the fluorocarbon's temperature: the colder the fluid, the lower the respiration rate which in turn prevented carbon dioxide buildup. He therefore induced hypothermia in the animals. This technique seemed to give the most success, as one animal survived over 20 hours breathing fluid at 18oC.